Mastering the EU Joint Clinical Assessment (JCA): Your Path to EU Market Access
The new EU Health Technology Assessment (HTA) Regulation (EU 2021/2282) has fundamentally reshaped market access in Europe. The Joint Clinical Assessment (JCA), mandatory for oncology and ATMPs from January 2025, presents a critical challenge: a harmonized clinical assessment that demands strategic preparation and precise evidence.
At Sur Access, we specialize in navigating these complexities. We empower pharmaceutical and biotech companies to confidently prepare for JCA, ensuring their innovative therapies achieve optimal patient access across the EU.
The JCA Challenge: From PICO Complexity to Dossier Submission
The JCA process centers on addressing specific research questions framed within the PICO framework (Population, Intervention, Comparator, Outcome). While aiming for efficiency, this process introduces distinct challenges for Health Technology Developers (HTDs):
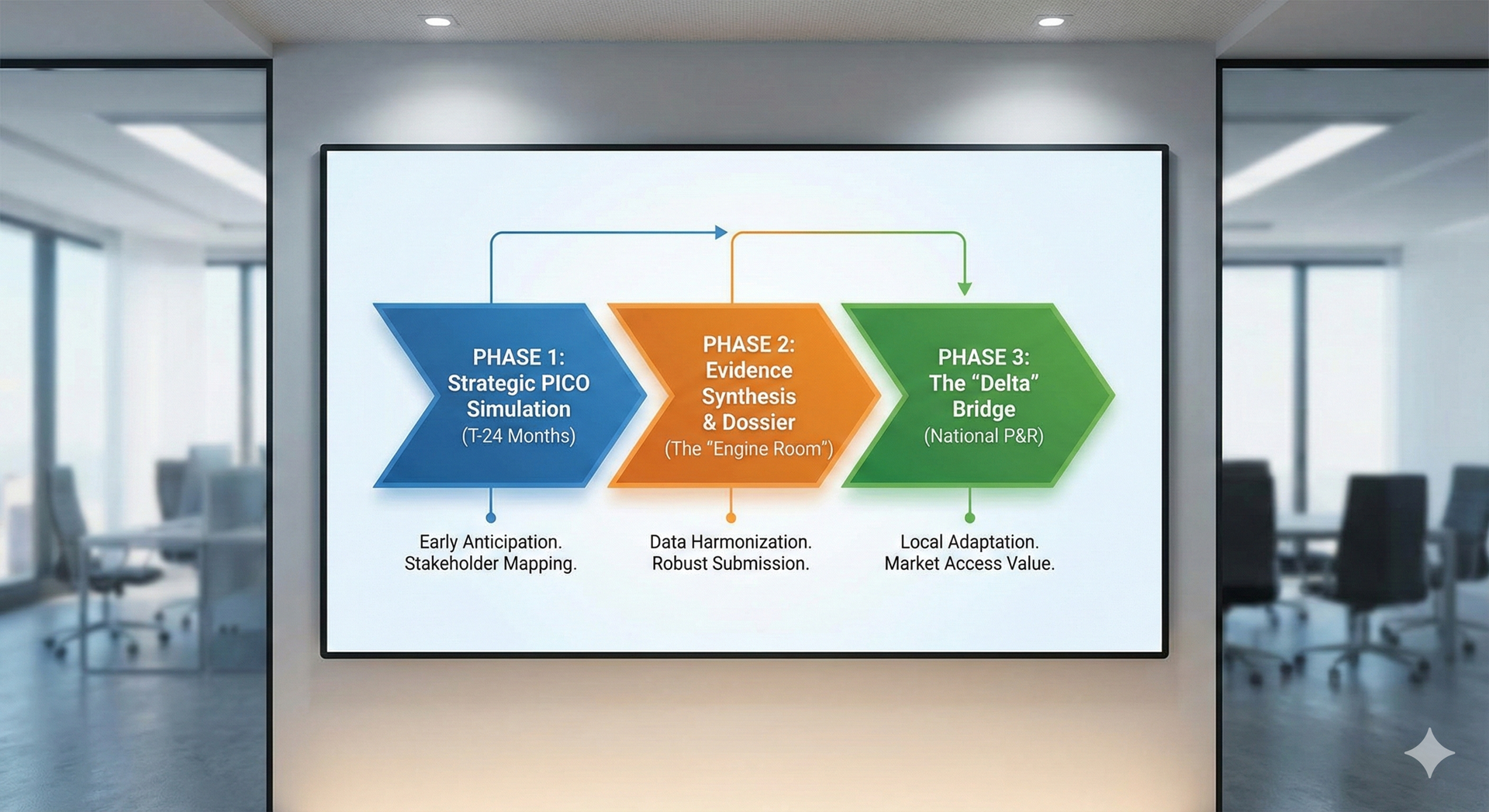
- PICO Proliferation & Diversity: Each EU Member State contributes to the PICO scope, often resulting in a substantial number of diverse PICOs (e.g., 10-30+ for oncology products). This necessitates anticipating a wide range of relevant comparators and patient subgroups, frequently beyond the scope of pivotal trials.
- Evidence Alignment & Gaps: Your existing clinical data must be strategically mapped and synthesized to address every defined PICO. Identifying and robustly addressing evidence gaps (especially for indirect comparisons) is crucial within tight timelines.
- Compressed Dossier Timelines: Once the final PICO scope is determined, HTDs face a short, intense window – typically 100 days (or 60 for accelerated procedures) – to compile and submit a comprehensive JCA dossier.
- Navigating National Nuances Post-JCA: The JCA report is a clinical assessment and non-binding. National HTA bodies still conduct their own pricing and reimbursement (P&R) assessments, potentially requiring additional "delta" dossiers or specific economic analyses.
Our JCA Services: Strategic Solutions for Every Step
Leveraging our deep expertise and our network of partners, we offer targeted services designed to streamline your JCA journey and optimize your European market access strategy:
- PICO Anticipation & Strategy Development:Proactive PICO Simulations: Conducting early analyses to predict likely PICO demands across Member States, enabling you to align your Phase 3 clinical trial design and evidence generation strategy effectively (ideally 18-24 months prior to EMA submission).
- Comparator Identification & Justification: Identifying all relevant direct and indirect comparators across the EU, and developing robust justifications for your chosen comparison strategies within the PICO framework.
- JCA Dossier Development & Optimization:Strategic Dossier Planning: Guiding the architectural planning and content strategy for your JCA dossier to address all defined PICOs efficiently and persuasively.
- Evidence Synthesis (ITCs & NMA): Applying advanced methodologies, including Indirect Treatment Comparisons (ITCs) and Network Meta-Analysis (NMA), to robustly address evidence gaps for indirect comparisons against relevant comparators identified in the PICO scope.
- Clinical and Safety Narrative Development: Crafting compelling clinical and safety sections that highlight your product's value in line with JCA requirements.
- Post-JCA National HTA & P&R Support:JCA Report Dissemination Strategy: Advising on how to effectively integrate and leverage the published JCA report into your national HTA submissions.
- "Delta" Dossier Preparation: Supporting the development of supplementary evidence and country-specific economic analyses required by national P&R bodies to bridge any gaps between the JCA and local requirements.
- Stakeholder Engagement & Training:Providing strategic advice on engaging with the EU HTA Coordination Group, national HTA bodies, and other critical stakeholders throughout the JCA process.
- Offering targeted training sessions for your internal teams on JCA requirements and best practices.
Why Choose Sur Access for Your JCA Journey?
The EU HTA Regulation demands a sophisticated, integrated, and proactive approach. Partnering with us ensures:
- Deep JCA Expertise: Direct access to our specialized knowledge in HTA, rare diseases, and the evolving EU framework, informed by our role in the HTAi Rare Disease Interest Group.
- Tailored & Agile Solutions: We offer a personalized, hands-on approach that adapts quickly to your unique product and the dynamic JCA landscape, without the bureaucracy of larger firms.
- Integrated Support: Through our established network of regulatory and corporate establishment partners, we provide seamless, end-to-end solutions from legal entity setup in Switzerland to market access strategy and national reimbursement.
- Results-Oriented Focus: Our aim is to build robust, evidence-based dossiers that lead to successful JCA outcomes and optimal patient access across Europe.
Don't let JCA complexity delay your market access. Contact us today to discuss your specific needs.
